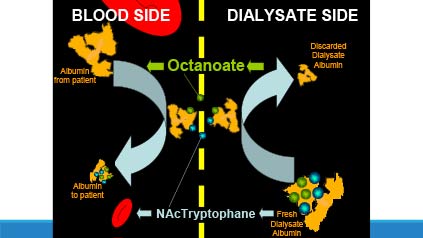
The ALIVER Consortium, which is comprised of experts in liver failure, small and medium-sized enterprises (SMEs) and not-for-profit organisations, proposes to perform clinical trials of DIALIVE in patients with acute-on-chronic liver failure (ACLF).
During the grant period, a “Conformité Européenne” (CE)-mark will be obtained and the device will progress to a TRL7/8. Consultation with regulatory bodies confirm that if the trials are successful, a CE-mark is highly likely. Grifols, a large plasma proteins company is a potential licensee of the technology if the studies proposed by the ALIVER Consortium are positive.
We plan to take the project through regulatory and ethics approval and perform two studies to define its safety and efficacy in ACLF patients in 18 European hospitals; define health economic benefits to the EU and define a reimbursement strategy.